Projects
Integrated Capsule Microsystems for in situ Biosensing and Diagnostics
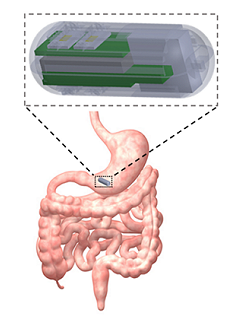
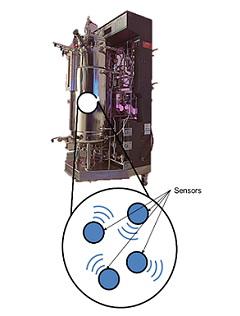
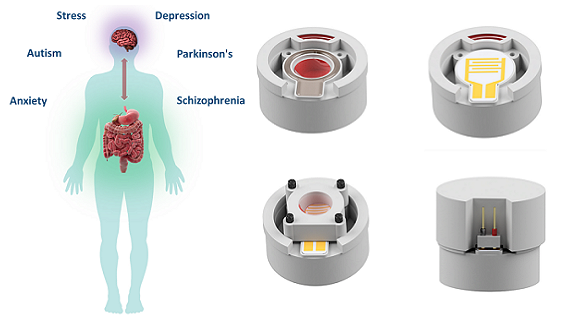
With the advancement of micro- and nano-technologies, miniaturized bio/medical devices and systems are becoming increasingly more viable for addressing challenges associated with the current state-of-the-art methodologies, which require complicated and expensive devices manned by trained personnel. Our group is taking a multi-directional approach developing smart miniaturized bio/chemical sensing platforms for in vivo diagnostics of GI tract, real-time monitoring of biological reactors and unveiling molecular cross talk occurring at the gut-microbiome-brain-axis. Electrochemical detection, is a simple yet powerful tool for molecular and cellular biosensing in aqueous environments. By encapsulating electrochemical electrodes along with battery and communications electronics, we aim to send these capsule type devices into biological environments (e.g. GI tract) where they can wirelessly transmit molecule biosensing data for external analysis toward better pancreatic adenocarcinoma (PA) diagnostics. We aim to develop capsules capable of detecting digestive enzymes and microRNA, characteristic of PA in pancreatic secretions present in the GI tract. To achieve this, we are integrating biomarker-specific sensors with off-the-shelf microelectronics, packaged within a 3D-printed capsule to protect our system while it wirelessly transmits signals to a mobile phone. Our long-term goal is to utilize this device as a preemptive screening tool for patients with potential for developing PA. This microsystem has the potential to be customized to sense and transfer useful diagnostic data on various events occurring in the gut. A larger scale version of an analogous system can be used to monitor the distribution of molecules of interest in a bioreactor. At these scales, it is critical to understand the biochemical profile of the environment. This tool will introduce a new level of sampling and quality control to various industrial bioreactor systems. A similar electrochemical sensing approach is also being used to design a lab-on-a-chip in vitro model of the gut, with integrated porous Impedimetric and electrochemical sensors for evaluating one’s gut-microbiome. This device would be used to diagnose the metabolic state of the gut microbiome in patients exhibiting both GI and neurological disorders and solve the missing links in molecular cross talk at the Gut-microbiome-brain-axis.
Micro-Nano-Bio-Systems Integration Using Biological Nanoscaffold for Sensing and Energy Harvesting
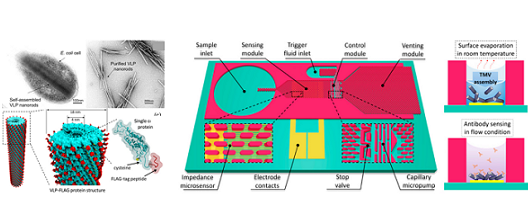
TVM-VLP's and their integration with microfluidics-integrated sensing platforem
for high performance biomolecular detection.
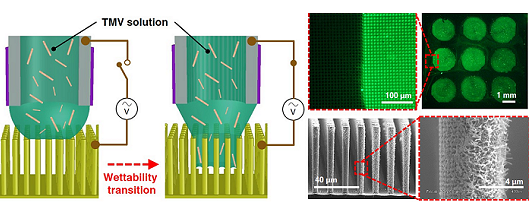
Development of a novel biopatterning technologies on three-dimensional device
substrated tailored towards development of advanced bioenergy harvesting devices.

Electron microscopic images of nanostructured energy storage electrodes created via integration of TMV nanoscaffolda with advanced micro/nanofabrication technologies.
The convergence between biochemistry and micro/nano manufacturing technologies has led to revolutionary advancements in the development of miniaturized devices and systems equipped with biochemical functions from integrated biomaterials (e.g. nucleic acids, antibodies, viruses, etc.). Particularly, viral nanoparticles that are non-infectious to humans (plant viruses and bacteriophages) have been vastly utilized as functional nanomaterials leveraging their self-assembled nanostructures, molecular storage functions, and high-density protein residues expressed on their surface. Over the past decade, the efforts by our group and collaborators have focused on combining genetic-engineering and microfabrication/MEMS technologies to unveil beneficial utilities of a specific plant virus, tobacco mosaic virus (TMV), and its gene-free derivative, virus-like-particles (VLPs), for developing a range of advanced nanomaterials and microsystems. TMV displays a cylindrical high-aspect-ratio structure formed by assembly of thousands of identical CPs encapsulating its genome. The bio-nanoscaffold serves as an excellent building block for synthesis/fabrication of nanomaterials for a range of applications including high performance charge storage devices (e.g. Li-ion batteries, supercapacitors) and superhydrophobic surface coatings. In addition, the naturally arranged high density surface receptors can offer versatile functions for developing advanced bioelectronics including bio/chemical sensors and bioenergy harvesting devices. We continue our efforts in developing methodologies to overcome fundamental challenges associated with integration of TMV/VLP particles with micro/nano devices, with the long-term aim of developing translational microsystems enabled by harmonic hybridization of bio-, nano-, and micro- components.
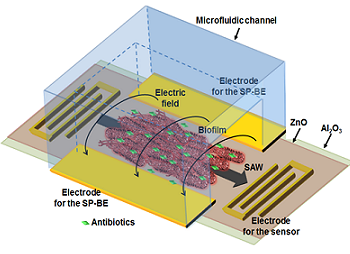
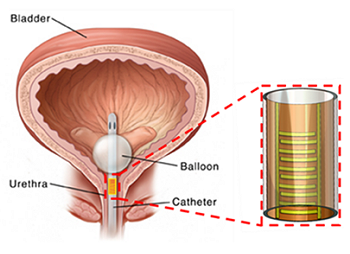
Micro/Nano Devices and Systems for Bacterial Biofilm Detection, Monitoring and Inhibition
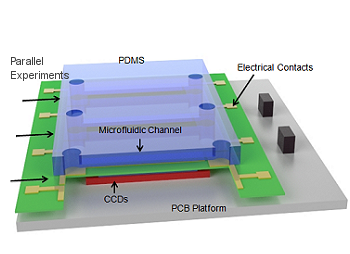
Microsystems with integrated biosensors are making a paradigm shift in the way biomedical devices are impacting the field of health care. Biofilms are ubiquitous in healthcare and is a leading cause of hospital associated infections (HAIs) causing catheter and implant associated infections. Biofilm consists of cells encased in a sticky polysaccharide extracellular matrix, forming when bacteria adhere to hydrated surfaces. Traditional research has focused on planktonic bacteria when developing therapies for treating infections. However, in reality, biofilms provide bacteria with elevated resistance to traditional antibiotics, rendering these treatments ineffective. Furthermore, biofilms and the higher doses of antibiotics used for treatment serve to exacerbate the spread of antibiotic resistance. In our group, we have developed several microsystems for effectively characterizing and treating biofilms. Microfluidic systems enable tight control of the biofilm microenvironment and minimize reagent volumes, allowing rapid high-throughput testing of the interactions of different compounds and conditions with biofilm. Also, these microfluidic systems can be designed with integrated controls to improve experiment reproducibility and minimize the impact of the inherent variability in biofilm growth. In addition, we have developed integrated microsystems combining the microfluidics with novel biofilm sensing and treating strategies. In particular, our recent efforts have demonstrated real-time monitoring of biofilm growth by measuring the biofilm-induced change in biomass or dielectric property using surface acoustic wave sensor or an impedance sensor. In addition, these sensors have been integrated with a biofilm removal mechanism, bioelectric effect, a phenomenon where a small electric field increases the susceptibility of a biofilm to an antimicrobial compound. Our current efforts are tailored towards developing flexible sensing platforms to allow the integration of these sensing and treating systems with the complex geometries of relevant colonized surfaces in medical applications. Overall, the developed microsystems will allow us to examine novel treatment methods and minimize the spread of antibiotic resistant strains which would make a significant impact in healthcare.